Will You Be Ready to Use These New CPT® Codes in the New Year?

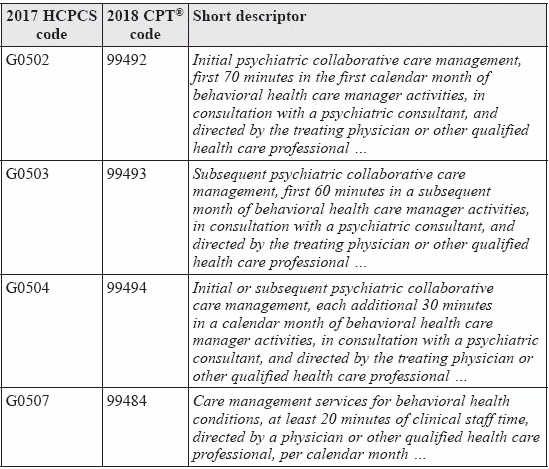
Jan 1. sees new behavioral health, cognitive impairment, and vaccine codes. 177 new codes. 60 revised codes. 79 deleted codes. The American Medical Association (AMA) has finalized its revisions for CPT® 2018. Will your practice be affected? Read on, and stay ahead of the changes that could impact your documentation and your bottom line in the upcoming year and beyond. New BHI Codes Replace Recent HCPCS Code Additions The most significant changes that will affect internal medicine practices are the following new codes for behavioral health integration (BHI) and behavioral health care management services: Background: In January 2017, the Centers for Medicare and Medicaid Services (CMS) introduced four HCPCS codes that "CMS created for 2017, knowing that the CPT® codes would not be available until 2018," according to Kent Moore, senior strategist for physician payment at the American Academy of Family Physicians. Moore goes on to explain that "three of these codes describe a particular model of BHI, known as a psychiatric collaborative care model (CoCM) that is not yet common in internal medicine, although some practices provide it." Looking ahead: Regarding 99484, Moore notes that "Because it is more generic, I expect that it will get more use by FPs." And Jan Blanchard, CPC, CPMA, pediatric solutions consultant at Vermont-based PCC, adds a note of caution regarding 99484 and all the other new BHI codes. "CPT® guidance clearly states that these services are provided for a 'diagnosed psychiatric disorder.'" Blanchard warns. "So, be clear about exactly what that means to your carriers and contact them for a list of specific ICD-10-CM diagnosis codes to discover whether they restrict the diagnoses for which they will pay." Replacement, Changes for HCPCS Cognitive Impairment Assessment Code CPT® will also add 99483 (Assessment of and care planning for a patient with cognitive impairment ...), replacing HCPCS code G0505 (Cognition and functional assessment ... for the patient with cognitive impairment ...). However, in the migration, CPT® adds several clinical responsibilities not spelled out in the original HCPCS code, including, but not limited to: New Flu Vaccine Codes Make Their Debuts ... In the midst of the 2017-2018 influenza season, 90682 (Influenza virus vaccine, quadrivalent (RIV4), derived from recombinant DNA, hemagglutinin (HA) protein only, preservative and antibiotic free, for intramuscular use) joins 90756 (Influenza virus vaccine, quadrivalent (ccIIV4), derived from cell cultures, subunit, antibiotic free, 0.5 mL dosage, for intramuscular use) as new additions to CPT® 2018, "continuing the revisions started last year to focus CPT® descriptors on dosage while discontinuing age specificity," according to Blanchard. Technically, neither code is new. Code 90682 made its appearance on the AMA web site on July 1, 2016, and became effective January 1, 2017, but 2018 marks the first year it will appear in the CPT® book. And the American Academy of Pediatrics (AAP) has noted that some payers began accepting 90756 - known by its trade name, Flucelvax - on July 1, 2017. But if your payer is not recognizing the code, you can use 90749 (Unlisted vaccine/toxoid) or HCPCS code Q2039 (Influenza virus vaccine, not otherwise specified) in its place. ... and a Shingles Vaccine Does, Too You'll also be able to document 90750 (Zoster (shingles) vaccine (HZV), recombinant, sub-unit, adjuvanted, for intramuscular injection) after the first of the year if your provider administers this vaccination to help prevent an occurrence or reoccurrence of shingles in patients over the age of 60. According to CPT®, Food and Drug Administration (FDA) approval of this vaccine is pending.