Preview Your Code Changes for Lab and Pathology With These 5 Highlights

The waiting is over — the AMA has released its preproduction version of the updated CPT® code set, so let’s take a look at changes you’ll face for your lab or pathology practice beginning January 1.
Read on to get an overview of what you can expect next year, and stay tuned to future issues of Pathology/Lab Coding Alert for in-depth analysis of these code changes.
1. Greet New OB Panel Code
CPT® 2016 adds a new option for billing an obstetric panel — an option that includes screening for human immunodeficiency virus (HIV) infection. Here’s the new code:
80081 — Obstetric panel (includes HIV testing)
This panel must include the following:
Blood count, complete (CBC), and automated differential WBC count (85025 or 85027 and 85004)
OR
Blood count, complete (CBC), automated (85027) and appropriate manual differential WBC count (85007 or 85009)
Hepatitis B surface antigen (HBsAg) (87340)
HIV-1 antigen(s), with HIV-1 and HIV-2 antibodies, single result (87389)
Syphilis test, non-treponemal antibody; qualitative (e.g., VDRL, RPR, ART) (86592)
Blood typing, ABO (86900)
AND
Blood typing, Rh (D) (86901)
This code varies from the current OB panel code, 80055 (Obstetric panel...) only in the addition of the 87389 test. Next year, labs may perform either 80055 or 80081, depending on physician orders.
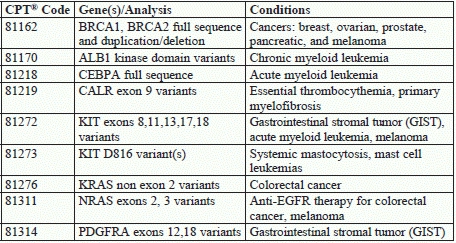
2. Anticipate Molecular Code Changes — But Wait for Payment News
You’ll have nine new Tier 1 molecular pathology codes next year to aid in genetic counseling, diagnosis, and treatment for the following conditions:
As with many molecular pathology tests that take time and analysis before coverage determinations appear, “we shall see what payers say about coverage,” cautions Melanie Witt, CPC, COBGC, MA, an independent coding consultant in Guadalupita, N.M.
Revisions happen: CPT® 2016 revises a few Tier 1 codes, primarily to update the gene name to more current nomenclature. But you’ll also see revisions to Tier 2 molecular pathology codes 81401-81406 (Molecular pathology procedure, Level [2-7] …). Most of the changes involve removing a listed test that becomes a new Tier 1 test this year, or adding a test to the specific Tier 2 service level.
3. Look for Genomic and MAAA Update, Too
You’ll find several revised codes in the “Genomic Sequencing Procedures and Other Molecular Multianalyte Assays” section. Most of the changes involve updating specific listed genes to better align with current protocols. Some of the changes are “housecleaning” issues, like stating that the codes include RNA analysis, “if performed.”
The more significant changes to the “genomic” section involve adding several new panels, as follows:
Manage MAAAs: Expect 10 new codes for multianalyte assays with algorithmic analyses, or MAAAs. These procedures involve running a panel of tests and using an algorithm to analyze the results (possibly with other patient data) to provide a risk or prognostic “score.” The new tests include the following options:
4. Update Infectious Organism Codes
You’ll see a CPT® 2016 systematic change to codes for infectious agent antigen detection by immunoassay technique (parent code 87301 through indented code 87430, and parent code 87449 through indented code 87451). The change appears in the common portion of the code descriptors, and simply provides examples of immunoassay technique, such as enzyme immunoassay (EIA), enzyme-linked immunosorbent assay (ELISA), immunochemiluminometric assay (IMCA). “The change shouldn’t alter how you use these codes,” says William Dettwyler, MT AMT, president of Codus Medicus, a laboratory coding consulting firm in Salem, Ore.
Also expect some minor revisions to influenza virus codes 87502 and +87503 for infectious agent antigen detection by nucleic acid, to include multiplex reverse transcription “when performed.”
Finally, CPT® 2016 makes minor revisions to the wording of immunology codes 86708-86709 for Hepatitis A antibody. The changes shouldn’t alter how you use the codes.
5. Renovate Immunofluorescence Coding
One code revision, one code deletion, and one code addition — that’s how CPT® 2016 revamps coding for immunofluorescence studies that your pathologist might perform for tissue or cellular specimens. The existing two-code structure distinguishes codes 88346 (Immunofluorescent study, each antibody; direct method) and 88347 (… indirect method) based on whether the method is direct or indirect immunofluorescence.
Beginning January 1, you’ll report immunofluorescence by either direct or indirect methods using two codes — revised code 88346 for “initial single antibody stain procedure,” and new code +88350 for “each additional single antibody stain procedure.” CPT® 2016 deletes 88347.
Antibody, rubella (86762)
Antibody screen, RBC, each serum technique (86850)