Keep On Top of These COVID-19 Code Choices
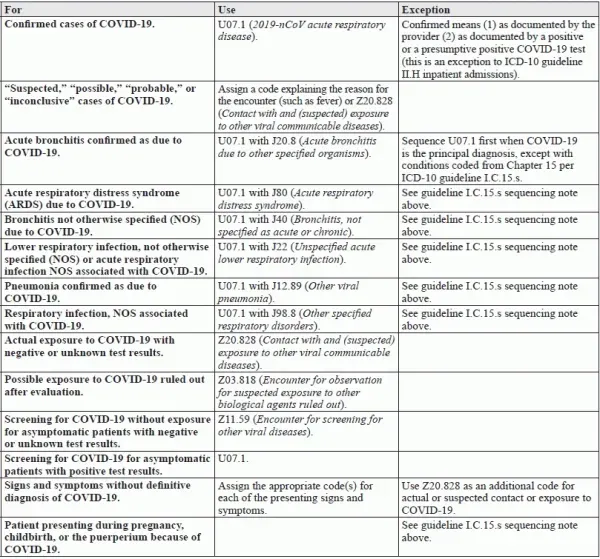
Answer: The Centers for Disease Control and Prevention (CDC) issued the following ICD-10 guidelines for COVID-19 effective April 1, 2020 through Sept. 30, 2020: On March 26, the American Medical Association (AMA) provided the following guidance for CPT® coding: For patient assessment: Use 99201-99215 (Office or other outpatient visit for the evaluation and management of a new/established patient …) either face-to-face or via telehealth; 99441-99443 (Telephone evaluation and management …), G2010 (Remote evaluation …), G2012 (… virtual check-in …) for telehealth; and 99421-99423 (Online digital evaluation and management service …) for virtual check-ins. For swab collection with in-person E/M assessment: Included in the evaluation and management (E/M) code. For swab collection without in-person E/M assessment: Use 99211, appending modifier 25 (Significant, separately identifiable evaluation and management service …) if this occurs in the provider’s office on the same day as a separate assessment that is not done in-person. Use 99000 (Handling and/or conveyance of specimen for transfer from the office to a laboratory) if your office incurs expenses preparing the specimen or is charged for the courier picking up the specimen to be delivered to the lab. For specimen collection at an independent testing site, the AMA recommends coding 99001 (Handling and/or conveyance of specimen for transfer from the patient in other than an office to a laboratory (distance may be indicated)). If the collection is bundled into the lab fee, then you cannot use 99000. Medicare carriers particularly consider collection and handling of specimens as part of an E/M service, and you should not code for it separately. For lab tests: Use 87635 (Infectious agent detection … [COVID-19]…) for dates of service on or after March 13, 2020. Use temporary codes U0001 (CDC 2019 Novel Coronavirus (2019-nCoV) …) for CDC laboratories for dates of service on or after Feb. 4, 2020. Otherwise, use U0002 (2019-nCoV Coronavirus…) for dates of service on or after Feb. 4, 2020 until March 13, when you should report 87635. (Source: www.ama-assn.org/system/files/2020-03/cpt-reporting-covid-19-testing.pdf.) Remember, this advice was accurate at the time of writing, but information related to COVID-19 is changing rapidly. Please check the sites listed in this issue’s first article frequently to stay informed of the current situation.