Look Out: Some Phase 2 Regs On Hold
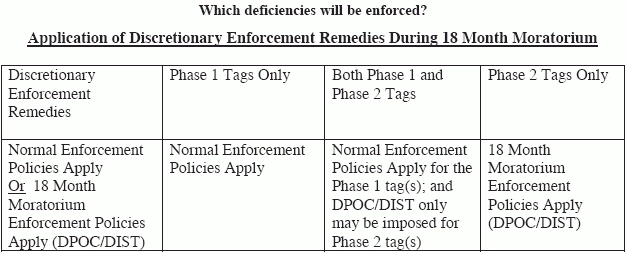
A last-minute CMS memo outlines some Ftag enforcement delays, as well as changes to Nursing Home Compare. With huge changes afoot in federal regulations of the long-term care industry, everyone is scrambling to adjust. The Centers for Medicare and Medicaid Services (CMS) is granting a pause of sorts, which may be just the breather you need to get all of your ducks in a row as the new survey process begins. Why the pause? Some Ftags enforcements are on hold for now, as CMS expands its window for getting surveyors ready for the new long-term care survey process. "CMS will use this 18-month moratorium period to educate surveyors and the providers to ensure they understand the health and safety expectations that will be evaluated through the survey process since these Phase 2 requirements are associated with unique and separate tags where specialized efforts and technical assistance may be needed," says David Wright, director of CMS's Survey and Certification Group in Baltimore, in a Nov. 24, 2017, memorandum to state survey agency directors. Which Ftags are affected? Wright says there's an 18-month moratorium "on the imposition of civil money penalties (CMPs), discretionary denials of payment for new admissions (DPNAs) and discretionary termination where the remedy is based on a deficiency finding of one of the specified Phase 2 Ftags," which includes the following: However, even though monetary penalties may not be given, surveyors may still cite facilities for these dificencies, says Marilyn Mines, Rn, BC, RaC-Ct, senior manager at Marcum LLP in Deerfield, Illinois. And don't get too excited. Providers won't be spared some of the more burdensome Phase 2 requirements of participation, says Linda Elizaitis, president and CEO of CMSCompliance Group in Melville, New York, in a recent blog post. "The Ftags that are part of the moratorium on remedies will still be cited as appropriate by state agencies and sent to the regional office as normal. Deficiencies cited under all other F-tags will follow the standard enforcement procedures." What about Five-Star Ratings? CMS waved the moratorium magic wand over Nursing Home Compare and Five-Star Ratings, too, Wright says. CMS will pause star ratings as the new long-term care survey process is rolled out. Wright says that he expects that most facilities will be evaluated using the new protocol by Nov. 28, 2018, but CMS will "hold constant" on the "inspection star rating for health inspection surveys and complaint investigations conducted on or after Nov. 28, 2017." Note: "Recent health surveys and complaint investigations conducted before Nov. 28, 2017, will continue to be calculated in a facility's star rating," Wright says, "including any revisit or changes based on informal dispute resolutions (IDR) or independent IDR." Other changes on Nursing Home Compare CMS is introducing summaries of facilities' most recent survey findings, Wright says. These summaries will include tidbits like the total number of deficiencies surveyors cited, as well as the highest scope and severity level. CMS plans to send a special shout-out to deficiency-free facilities, too. "We also will post the full report of each survey (Form CMS-2567), which provides more details about the survey findings," Wright says. "We expect to implement these changes in early 2018."