Closer Look: What To Expect From Upcoming MDS 3.0 Revisions
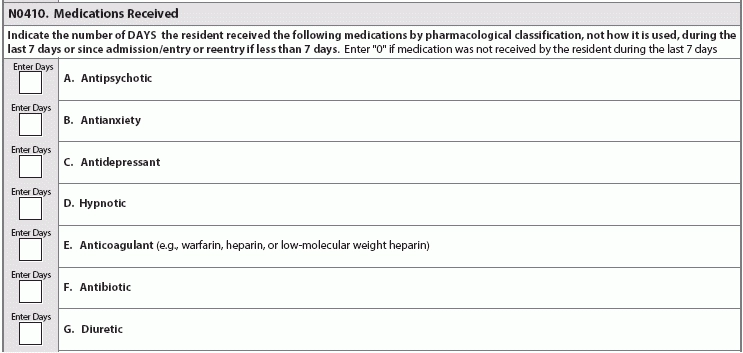
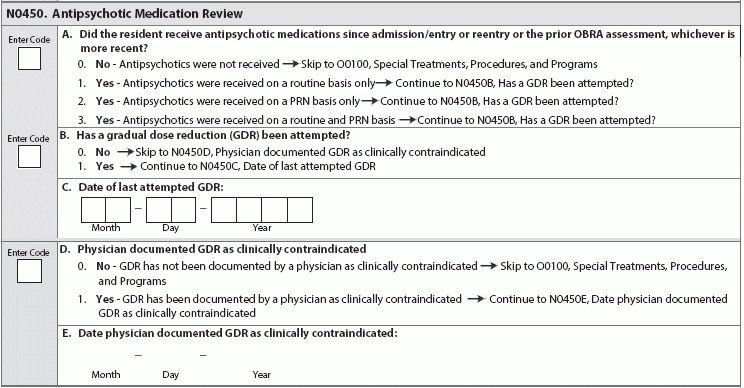
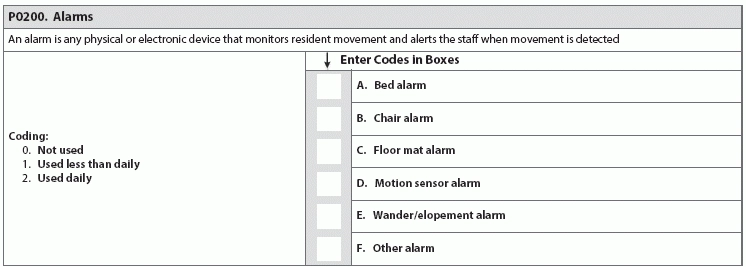
Find out why industry experts are puzzled by the new item P0200. Looking ahead to the upcoming MDS 3.0 revisions for October 2017, the good news is that the Centers for Medicare & Medicaid Services (CMS) hasn’t added many new items and has released the draft changes far in advance. But the not so good news is that the MDS additions will require tracking and monitoring. CMS released the draft MDS 3.0 version 1.15.0 item sets back in December 2016 (see “Get A Sneak Peek At Draft MDS 3.0 Item Set Changes For 2017,” MDS Alert, Vol. 14, No. 12, page 142). The draft MDS includes several new items, some of which are tied to the revisions to the Requirements of Participation (RoP), particularly the Phase 2 mandates (see “5 Crucial Facts You Need To Understand About The Baseline Care Plan” below). Reaction: Recent MDS 3.0 changes have “evoked anxiety in the industry, not totally because of the elements added, but because of the time constraints and resources required to complete the MDS,” notes Kris Mastrangelo, President and CEO of Harmony Healthcare International. “For example, the MDS Coordinator must complete Section G and Section GG,” Mastrangelo points out. “Both areas code Activities of Daily Living, yet both areas are required for different reasons (RUG ADL End Split and QRP Data, respectively). The content is duplicative and it frustrates the staff because ‘time’ is what everyone needs.” Don’t Be Surprised By New Opioids Item The first major addition to the draft MDS for publication in October 2017 is a new sub-item under N0410 — Medications Received: N0410H — Opioids. “The addition of Opioids was definitely expected given the epidemic issue across the country,” Mastrangelo says. “The antipsychotic medication review was also predictable given the focus on overall medication management and contraindications.” “With the national problem of opioid abuse sweeping our country, it is not a surprise to see this drug classification added to the MDS 3.0,” agrees Jane Belt, Ms, Rn, RaC-Mt, QCP, Curriculum Development Specialist for the American Association of Nurse Assessment Coordination (AANAC). The U.s. Centers for Disease Control and Prevention (CDC) has released “Guidelines for Prescribing Opioids for Chronic Pain” (see www.cdc.gov/drugoverdose/epidemic), “as it has been found that there actually is safer, more effective chronic pain treatment to reduce the number of people who misuse, abuse or overdose,” Belt notes. Caveat: Opioids do have a place for use in the long-term care setting, such as in the case of residents who are actively dying and those with complex chronic pain syndromes (defined as greater than three months), Belt points out. “But opioids should not be prescribed as the first-line treatment for chronic pain.” Typically, a resident is admitted from the acute-care setting after having surgery or some complex medical condition, and opioids have been part of the treatment plan. The challenge in the long-term care setting is determining the dosing needed based on the drug’s effectiveness and the potential for adverse consequences. You can certainly anticipate adverse consequences to analgesics, and you can also prevent or reduce them to some extent. For instance, opioids routinely cause constipation, which you can minimize by appropriate bowel regimen. Best: Monitoring the use of opioids on the MDS “will provide additional data and a stimulus to the nurse to remind the physician or prescriber if it may be time to make a change in the treatment plan,” Belt says. “Pain certainly does need to be managed, but in a safe and effective manner and relative to the resident’s goals.” How & When to Code N0410H You will find the added opioid item on nearly all the MDS item sets. The new item N0410H appears on the following: o Comprehensive (NC) o NH PPS (NP/PPS) The only change to item N0410 is the addition of opioids, Belt says. The item’s overall coding and instructions are the same, stating that you must “indicate the number of DAYS the resident received the following medications by pharmacological classification, not how it is used, during the last seven days or since admission/entry or reentry if less than seven days. Enter ‘0’ if medication was not received by the resident during the last seven days.” How New N0450 Item Links to RoPs The second significant addition is the new item N0450 — Antipsychotic Medication Review. The addition of this new item “is not surprising based on the efforts by CMS to reduce unnecessary antipsychotics,” Belt notes. But the new item N0450 also has direct ties to the RoP revisions, particularly the changes to §483.45 Pharmacy Services. In Phase 2, the pharmacist will need to do a monthly review of the medical record. This will include a drug regimen review (DRR) and issuing a written report to the attending physician, director of nursing and medical director, noting any irregularities. Then, the attending physician must document in the medical record that an identified irregularity was reviewed and what, if any, action taken, Belt explains. If there are no changes made to the course of treatment, the attending must document the rationale. Also, your facility will need to develop policies and procedures for the monthly DRR in coordination with the pharmacist and the physicians. Your policies and procedures must include time frames for steps in the process, as well as the steps the pharmacist must take when she identifies an irregularity that requires urgent action to protect the resident. Pay attention: For years, facilities have been bound by the regulations regarding gradual dose reduction (GDR) for antipsychotics, “but now every OBRA MDS will need to have the actual date of the last attempted GDR and, if the reduction was contraindicated, the date that occurred,” Belt states. “Knowing surveyors, they will likely use that date to review the documentation to see if it is complete and clearly indicates the reasons for the attending’s action.” Beware: Additionally, what CMS considers a psychotropic drug will soon change, specifically in terms of limiting the length of time that you can use or order PRN medications. As of Nov. 28, 2017 (when Phase 2 becomes effective), “psychotropic medications will include antipsychotics, anti-anxieties, antidepressants, and hypnotics,” Belt says. PRN orders for these medications will be limited to 14 days, unless rationale is documented and the new duration is specified. Therefore, PRN orders for antipsychotics will be limited to 14 days and cannot be renewed unless the attending physician evaluates the resident. Alarms: To Use or Not to Use? Another major addition is the new item P0200 — Alarms, which tracks the use of chair, floor mat, motion sensor, wander/elopement, and other alarms. This new item has caused some confusion, specifically when it comes to the meaning behind the item. Problem: “Alarms are tricky because, when implemented years ago, the intent was safety,” Mastrangelo notes. “Then, the studies showed that the constant beeping is tuned out by the caregiver (hence ineffective), yet the noise eventually agitates everyone in the area. This is a reminder of good intent but not so good impact.” The addition of the new item P0200 “surprised me as alarms have been indicated by CMS in 2009 as something to eliminate to make the environment more home-like,” Belt says. “Since we do not have all the new interpretive guidelines, there may be more information to come, but I see it as a way to easily track alarm use and for surveyors to determine appropriateness and effectiveness since the research shows that alarms do not reduce falls.” Tricky: And the guidance in the revised RoPs doesn’t help much, as some parts seem to suggest the use of alarms, while other parts indicate a need to eliminate them, Belt points out. “I believe the word to the wise here is CMS will be looking for elimination of personal alarms. Tracking their use via the MDS is a start to their data analysis.” Strategy: Stay Ahead of the Regulatory Curve “There is a definite concern (or fear) that items are added for ‘data mining’ versus care planning, quality measures or reimbursement,” Mastrangelo cautions. Nevertheless, “the industry is moving fast in response to the abundant regulatory changes.” “The government is researching and implementing appropriate changes in the best interest of the residents,” Mastrangelo continues. “However, this pace requires funding in order for the providers to implement the necessary systems to abide by the additional regulatory requirements and meet the expectations of the newly defined quality of care metrics.” Bottom line: Keep in mind that these are still just draft changes and won’t become finalized until closer to the Oct. 1, 2017 effective date. To preview the draft MDS item sets and data specifications, go to www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIMDS30TechnicalInformation.html.
o NH Discharge (ND, NOD)
o Quarterly (NQ)
o NH Start of Therapy/DC (NSD)
o SB Discharge (SD)
o Swing-bed PPS (SP)
o SB OMRA-Discharge (SOD)
o SB OMRA SOT/Discharge (SSD)