What Do You Think?
Question 1: The resident was admitted to skilled services on May 16 following an acute hospital stay for a hip fracture. But the resident discharged the same day and was readmitted to the hospital. Since this isn’t a billable day, are any assessments due?
Answer 1: Even though there won’t be any reimbursement, you still must follow Omnibus Reconciliation Act (OBRA) rules, according to the Texas Department of Aging and Disability Services (DADS). You must complete an Entry and a Discharge Assessment, even though much of the information on the assessments will be dashes.
Remember: These types of assessments are the only way that the Centers for Medicare & Medicaid Services (CMS) can track a resident in the various levels of care, DADS noted.
Question 2: When do I need to complete the Part A PPS Discharge Assessment?
Answer 2: CMS recently added a third type of discharge assessment — the Part A PPS Discharge Assessment. The first thing you need to know about this discharge assessment is that you don’t complete it when the discharge is unplanned.
Right way: Instead, you complete this assessment “when a resident’s Medicare Part A stay ends, but the resident remains in the facility,” states the Kansas Department for Aging and Disability Services (KDADS). “It is also required when a resident is physically discharged on the same day or within one day of the end of the Medicare Part A stay.”
“When this happens, the OBRA Discharge Assessment and Part A PPS Discharge Assessment are both required and may be combined,” KDADS notes. “If Medicare ends on the same day the resident expires, a Death in Facility Tracking Record is completed, but a Part A Discharge Assessment is not required.”
Scenarios: If the last day of the resident’s Medicare Part A benefit is before Day 7 of the Change of Therapy (COT) observation period, then you don’t need to complete a COT Other Medicare Required Assessment (OMRA). If the last day is on or after Day 7, you must complete a COT OMRA if all conditions are met.
When the Medicare Part A stops on Day 7 of the COT observation period, then you must complete both the COT OMRA and the Part A PPS Discharge Assessment separately.
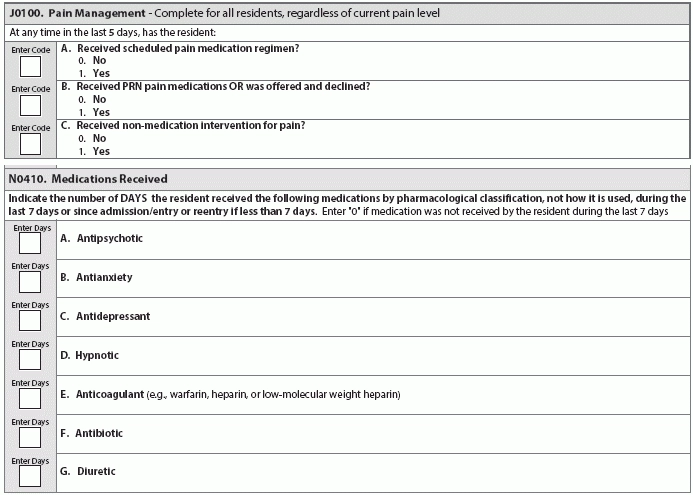
Question 3: Can I code pain medication in J0100 even if the drug isn’t an analgesic?
Answer 3: Item J0100 — Pain Management documents whether a resident received pain medication and/or non-medication interventions for pain. To understand what you should code for this item, you can first look to the sub-item J0100A — Received scheduled pain medication regimen?
To properly answer this question in J0100A, you need to look to the RAI Manual’s definition of “pain medication regimen,” according to the Oklahoma State Department of Health (OSDH) Quality Improvement & Evaluation Service (QIES). The RAI definition is as follows:
“Pharmacological agent(s) prescribed to relieve or prevent the recurrence of pain. Include all medications used for pain management by any route and any frequency during the look-back period. Include oral, transcutaneous, subcutaneous, intramuscular, rectal, intravenous injections or intraspinal delivery. This item does not include medications that primarily target treatment of the underlying condition, such as chemotherapy or steroids, although such treatments may lead to pain reduction.”
Notice that the definition does not include that the pain medication be an analgesic, OSDH QIES points out. “When a medication is given routinely to manage pain (even if it is not an analgesic), and the medical record contains documentation that the scheduled medication is being given to manage pain, then the facility may code that the resident received a scheduled pain medication.”
Note: Coding for N0410 has been the source of enough confusion that CMS recently revised the instructions for the new RAI Manual version (effective Oct. 1, 2016) to read: “Indicate the number of DAYS the resident received the following medications by pharmacological classification, not how it is used, during the last 7 days…”
Question 4: Should I code medications like Plavix, Pravigard, Pletal (cilostazol), and aspirin in N0410E?
Answer 4: No, you should not code these types of medications in Item N0410E — Anticoagulant, because these medications are antiplatelet drugs, not anticoagulants.
Confusing the two types of drugs when coding N0410E is easy, because both anticoagulant and antiplatelet drugs are classes of antithrombotic drugs, routinely used to treat and prevent blood clots, and also commonly known as “blood thinners,” says Katy Nguyen, MSN, RN, an educator with the Quality Improvement Program for Missouri’s Long-Term Care Facilities (QIPMO), a cooperative program between the MU Sinclair School of Nursing and the Missouri Department of Health and Senior Services.
But in N0410E, the item is specifically asking for anticoagulants. This is because anticoagulants require more intensive monitoring processes with laboratory testing, while antiplatelet drugs can require a less intensive monitoring process from nursing care, observation, screening, and assessments, Nguyen explains.
Do this: According to Nguyen, you should code the following common anticoagulants in N0410E:
Mistake: But do not code the following most common antiplatelet agents in N0410E: