Prepare Now For Section N And Section P Additions Or Pay The Price
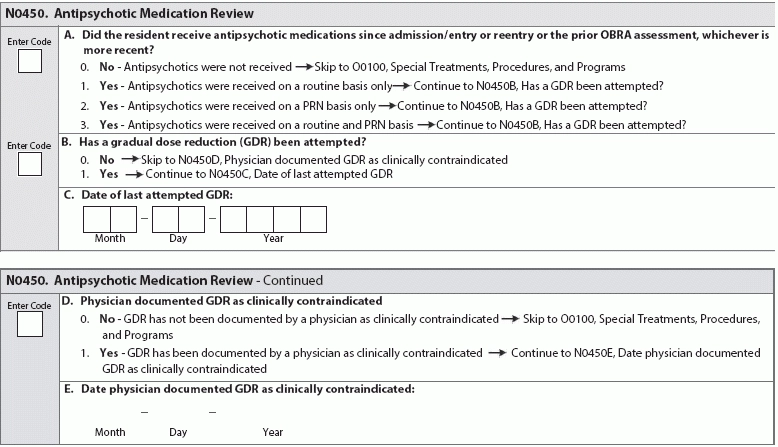
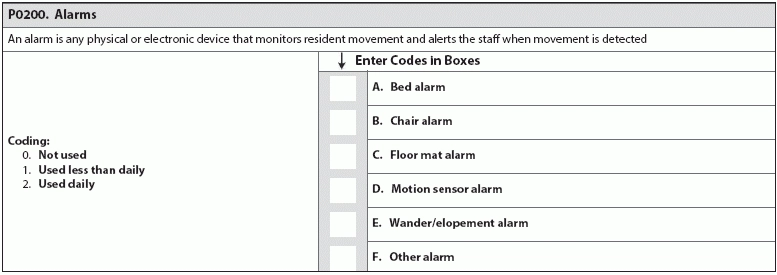
Bolster your knowledge and adjust your protocols before changes go into effect. Oct. 1, 2017, is fast approaching; don’t fall behind on your evaluating and coding the new MDS item sets. The additions to Section N and Section P are topical and a nod to the sea change toward more patient-centered care. Remember: Make sure you’re prepared for the requirements to code these new MDS items now, before MDS 3.0 is updated in October 2017. Hint: Your values for coding the new sub-item (Opioid) in item N0410 (Medications Received) are 0-7: the number of days a resident received medications in the seven-day lookback period. Sub-item N0410H (Medications Received … Opioids) requires coding the number of days a resident received opioids in the last seven days or since admission/entry or reentry. “The instructions for this item have not changed — the nurse or assessor must count the number of days in the lookback period that the resident received an opioid,” says Jane Belt, Ms, Rn, RaC-Mt, QCP, Curriculum Development Specialist at american association of nurse assessment Coordination (AANAC) in Denver, Colorado. When evaluating a resident’s medication regimen, especially new sub-item N0410H (Opioids), consider these RAI Manual tips: Prepare to Code and Review Antipsychotic Meds The other addition to Section N is item N0450 (Antipsychotic Medication Review), which requires documentation of antipsychotic medication use and provides space for you to record attempts at gradual reduction of antipsychotics dosage. Hint: This item is fairly unique in the MDS, in that there’s no specified lookback period. In this item, you’ll document how the antipsychotics are given (routine basis, PRN, or routine and PRN basis), whether GDR (gradual dose reduction) has been attempted, the date of the last GDR attempt, and whether a GDR is contraindicated. Watch for RAI Manual instructions in the MDS update in October 2017. Until then, refresh your knowledge on inappropriate indicators for usage of antipsychotic medications. Consider the following pointers from a 2013 workshop presentation by Lisa O’Hara, PharmD, CGP, Corporate Director of Clinical Services at Grandview Pharmacy in Brownsburg, Indiana. Inappropriate indicators for usage of antipsychotic medications include: Brush Up On Gradual Dose Reduction While the industry waits for specific instructions on N0450 (Antipsychotic Medication Review), use the next few months to build foundational habits that will help you code the new item successfully. Keep in mind: According to AANAC, a GDR may be clinically contraindicated if a resident is receiving antipsychotic medication treatment for a psychiatric disorder or behavioral symptoms. But you need to back up that assertion with evidence. The determination that a GDR is clinically contraindicated must be documented and supported in the clinical record, says Marilyn Mines, Rn, BC, RaC-Ct, Senior Manager at Marcum LLP, in Deerfield, Illinois. Don’t be Held Back by CMS Contradictions About Alarms Beware: The current CMS requirements on alarm use are contradictory depending on interpretation of the Requirements of Participation, and the contradictions could affect how surveyors dish out F-Tags. For example: Two F-Tags for alarm use, F252 and F222, are slightly contrary: F252 demands the elimination of “widespread and long-term use of audible (to the resident) chair and bed alarms,” but F222 suggests establishing a care plan that might include “equipping the resident with a device that monitors his/her attempts to rise.” Common sense suggests making sure your facility is as homelike and comfortable as possible while also minimizing resident safety risks. Don’t Fall Behind Make sure your facility is attempting to reduce restraint use, including alarms. “It is a common practice now in facilities to reduce the use of alarms as they have been shown not to reduce falls and they are not ‘home like,’ nor do they provide the resident dignity,” Belt says. Bottom line: Bolster your residents’ quality of life and your patient care by reducing your use of alarms. With these habits in place, you’ll be ready to avoid any related F-Tags throughout your facility before P0200 (Alarms) arrives. Reducing alarm use now will also make coding P0200 (Alarms) a piece of cake.